Let’s discuss edibles, as despite cannabis use continuing to rise, the general knowledge on edibles has been very lacking.
Processing
You probably don’t want your treats to taste of plant matter, chlorophyll being a quite bitter and nasty flavor. To avoid this we collect the trichomes, or else the cannabinoids themselves from the buds before making edibles. The typical methods of extraction are dry sifting, ice water hash, rosin, solvent extraction (BHO, PHO), and supercritical solvent extraction.
Dry Sifting – Dry Sift, if high grade, will leave very little residue. However, in mid to low grade dry sift, you will find it imparts a hashy, bitter flavor. Flavor being imparted isn’t necessarily a bad thing, as with tasty hash it can be complementary (keeping in mind the flavor once heated will not be the same as what you smell when sniffing the hash containers or smoking some of it). For most tasks it’s not really something we want, and further, many people don’t care for the flavor. When infusing dry sift into an oil, if it left sediment on the bottom it’s already imparted a hashy flavor. You can generally hide / complement this flavor with cinnamon, ginger, or peanut butter.
Ice Water Hash – It is going to take an even higher grade of expertly dried and cured IWE if, again, your goal is to impart the least possible hash flavour. In fact, IWE will impart more of a hashy flavour than most any other form of extract. Any residual moisture will separate from the oil (think oil and vinegar), leaving a layer on the bottom of your oil, and giving your oil a very strong distinct taste.
Rosin – This is a great choice if making just a few edibles, as it leaves less hashy flavor than any processing method that doesn’t make use of solvents. For the cleanest flavor, rosin dry sift.
Solvent / Supercritical Solvent – When done correctly, these are the easiest to infuse while leaving nothing behind. No sediment, means no hashy flavor, and the potency is a bit easier to calculate once tested, as it’s more homogenous (same case with rosin).
Solubility
Cannabinoids are not naturally soluble in water, but are soluble in lipids, and alcohol. This is why most recipe call for butter, ghee, or a plant/seed oil, due to the solubility of cannabinoids in fats, these can be infused easily after which point you can cook with them as if they were normal butter, ghee, ect. These are not the only way of course, just the most common. Let’s explore a more in depth look of the options you have.
Lipids:
Lipid are a go to for most of us in daily cooking, from frying, baking, sauteing, you name it! Lipids are hydrophobic, and includes fats, oils, waxes, phospholipids, steroids (like cholesterol), as well as a few related compounds. However, we are mostly concerned with the fats and oils. These can include but are not limited to Avocado Oil, Butter, Canola (Rapeseed) Oil, Coconut Oil, Corn Oil, Ghee (clarified butter), Grapeseed Oil, Margarine, Olive Oil, Palm Oil, Peanut Oil, Safflower Oil, Sesame Oil, Soybean Oil, and Sunflower Oil. So, what differences should you keep in mind?
Saturated Fats vs Unsaturated Fats
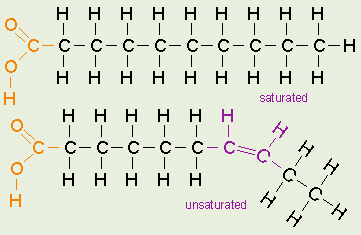
The chemical structure of saturated, and unsaturated fats. Picture by http://biologyclermont.info/
Fats are defined as either saturated or unsaturated based on the number of hydrogen atoms attached to the hydrocarbon tails of the fatty acids. A saturated fat has all single bonds between the carbon atoms in their fatty acid tails, and therefore all the carbon atoms are also bonded to the maximum number of hydrogen atoms possible. Because of the straight and tidy arrangement of the hydrocarbon chain in these fatty acids, these fats stay solid at room temperature. Saturated fats tend to be from animal sources of food, such as red meat, poultry, and full fat dairy products, whereas plant based sources generally yield unsaturated fats. These unsaturated fats have a double bond between some of the carbon atoms in the hydrocarbon tail, causing a kink in the shape of the molecule, as seen in the graphic. It is because some of the carbons are sharing a double bond, that they’re not bonded with as many hydrogen as they could otherwise. As well, they also can not pack as closely together, because of that kink, making them a liquid at room temperature.
In the solid state the individual triacylglycerol molecules are interacting with each other primarily through Van der Waals interaction. These weak bonds between molecules are broken at the solid-liquid transition. The amount of energy needed to disrupt these interactions (which determines the melting point of the fat or oil) is determined by the energy associated with all of these bonds added together. In a saturated fat the acyl chains are able to align perfectly right along their length, maximising intermolecular interactions. This effect is reflected in the fact that the melting temperature of a pure triacylglycerol increases as the chain length increases.
Alan Boyd
I know, I know, that was a lot of chemistry, but it’s not too hard to remember. Margarine is a partially hydrogenated vegetable oil (often soy oil), for example, and this should make more sense now, as it will melt on toast, however it’s not liquid like pure soy oil.
Why, you may be asking, does it being saturated vs unsaturated matter though?
The Health Impact
In the example above, we talked about how margarine is partially hydrogenated. If the hydrogenation is not completed, the high temperature the process takes place at tends to flip some of the carbon=carbon double bonds into the the trans position. This is what we refer to as a trans fat, and trans fats have been linked very clearly to cardiovascular health issues(1).
Saturated fat raises total blood cholesterol levels and low-density lipoprotein (LDL) cholesterol levels, which can increase your risk of cardiovascular disease. Saturated fat may also increase your risk of type 2 diabetes.
Mayo Clinic
The Taste Impact
For the reasons above, saturated oils can be a bit of a dirty word within health conscious circles. However, when it comes to flavor, their are some reasons saturated fats are still the go to for many cooks. For a very long time beef tallow was the go to oil for fast food french fries, however in 1990 many chains made the change over to an unsaturated oil. When they did, they immediately noticed they have to add tallow flavoring to augment their oil to make it still appealing. On the other side of the coin, if you were making potato chips you’d want to use an oil that melts in your mouth, which means the melting point of the oil has to be below body temperature. Otherwise, the chip will be unpleasantly tacky and greasy.
Most saturated oils, and even a few of the partially unsaturated oils, melt well above body temperature, so you’d not want to use them for fried foods that are to be served cold. Glazed donuts are a notable exception to this rule, as if you don’t want the glaze to crack you’ll need an oil that leaves a coating of solid fat at room temperature.
The stability of the oil also is incredibly important for flavor. Saturated oils are generally more stable than unsaturated oils, so they stay in peak frying condition longer, being that they are less prone to rancidity both in the oil, as well as the stored fried food. This is because in the unsaturated fats, the polyunsaturated molecules tend to cause the fat to degrade much more quickly as rancid aromas, and nasty compounds form.
Recommended Choices
After all that information, you may be wondering what inputs I’d personally recommend. My preference for baking / frying sweet things is coconut oil, and for more savory task I prefer avocado oil. Avocado Oil is high in monounsaturated fats, as well as vitamin E, and also enhances the absorption of carotenoids and other nutrients. (2) I’d first learned about the benefits of Coconut Oil for hash caps, and edibles from reading one of the best edible write ups of all time by BadKittySmiles, so I will share that section.
Coconut oil is ideal, because unlike most oil sources it is comprised mainly of MCT/MCFA, or medium-chain triglycerides/medium-chain fatty acids. The body accepts and absorbs these lipids much more easily than others, which are often partially passed right through, or are absorbed either too late, or too slow and less efficiently. Unlike other fatty acids and oils, MCT’s are delivered directly and with much greater ease to the portal vein, liver and blood stream. Their molecules are much smaller than Long-chain fatty acids, and therefore require less energy, and fewer enzymes, in order to become absorbed. It improves absorption and uptake of those nutrients and chemicals consumed at the same time, and it provides a source of high energy lipids to patients suffering from lipid digestion and absorption disorders such as pancreatitis and Crohn’s disease, and chylomicron deficiency.
People who suffer from certain ailments, need MCT’s regularly in their diets, in order to absorb almost everything, otherwise, they can become nutrient deficient regardless how healthy they eat, or how many supplements they take.
And in the same way it promotes absorption and helps those with digestive difficulty, it helps to an even greater extent, those with ‘normal’ and easy digestion, who can even more rapidly take in and absorb MCT’s than those who are ill.
BadKittySmiles
